Hantavirus: Ecology and Disease in US, Chile, and Panama
“The University’s Biology Department has been tracking [deer mice] for years – they had about a dozen years of data on the deer mouse population available at that point. (. . .) It was amazing how everything just fell into place. You think of what information it would be great to have, and it’s there!” – Bruce D. Tempest, M.D., 1998.
About 75% of all emerging diseases originate in wildlife, emphasizing the need to build comprehensive sample infrastructure to quickly and effectively respond to outbreaks. Museum collections play a critical, yet still underutilized, role in advancing our understanding of emerging pathogens by providing insights into the ecology, evolution, and transmission of new diseases that may emerge in new or shifting environments.
Natural history museums play a key role in documenting biodiversity, including pathogens and their hosts. Specimens from plants to mammals contribute vital data for pathogen research. Examples include:
- Yellow Fever
- Sin Nombre Hantavirus
- Helminth parasites
Still, there is a need to integrate museum collections into host pathogen research, as demonstrated with ebola viruses and the recent 2020 pandemic SARS-CoV-2 (COVID-19), both of which have unknown natural reservoirs.
Sample biorepositories, assembled over decades, provide researchers with fundamental data crucial for understanding how pathogens evolve, adapt, and spread:
- Species identity
- Distribution
- Interactions
- Geographic variation
Viral symbiotypes are the original host specimen for a newly described virus (Dunnum et al. 2017). MSB holds 18 new hantaviral symbiotypes, including:
- 9 non-rodent mammals, 7 from the United States, and 12 from international collaborations
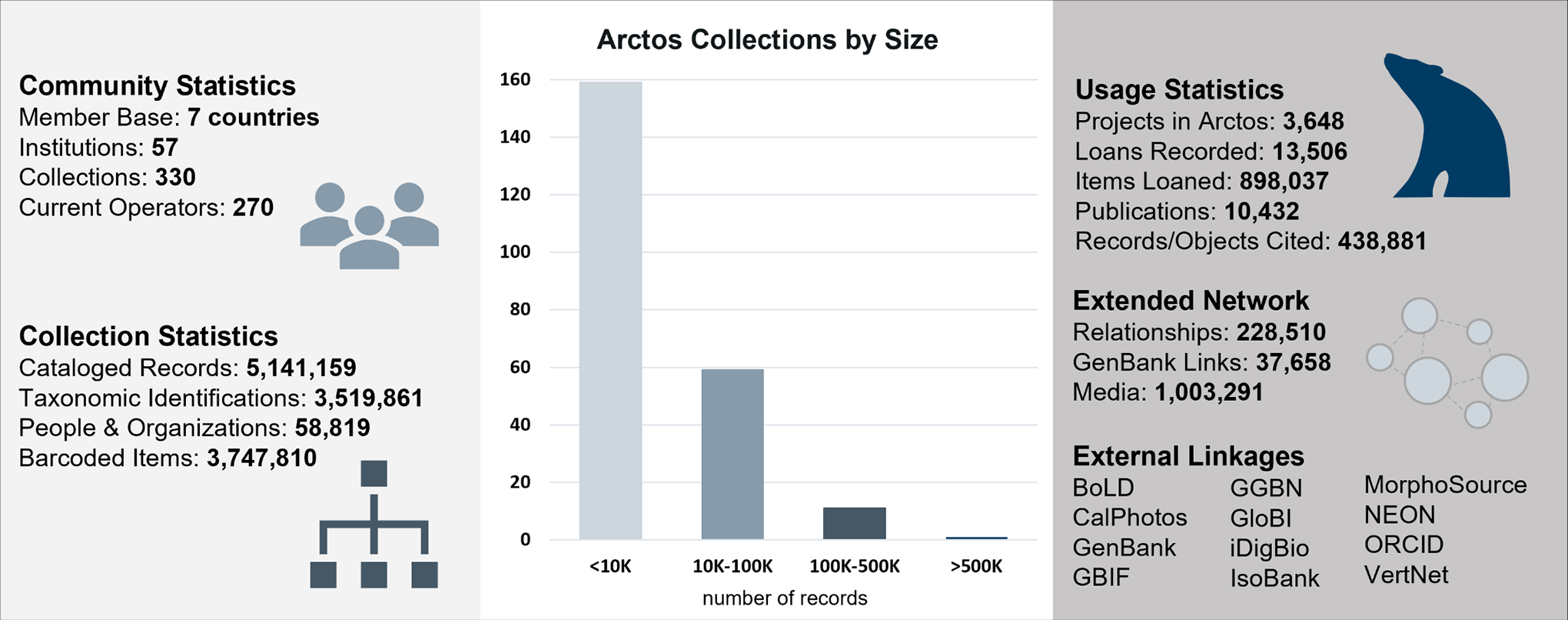
These specimens can be found through Arctos, a collection management database with >5 million records from ~330 collections. Arctos links specimens with each parasite or pathogen detected and provides direct links to GenBank, IsoBank, and other external sites.
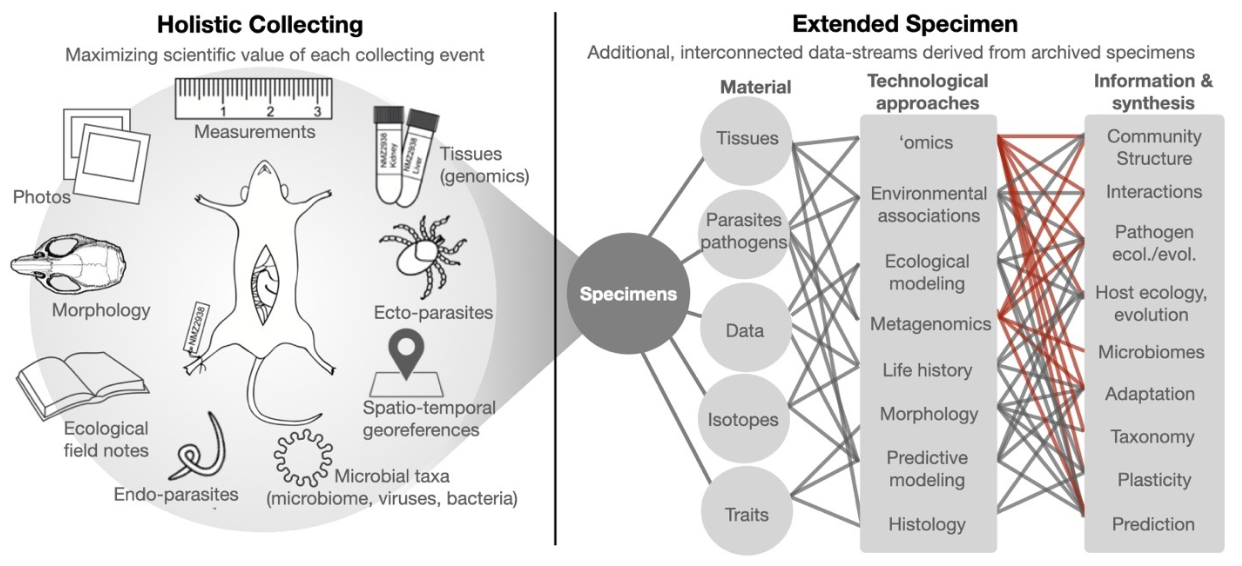
The MSB has reinvigorated field collection strategies by promoting the “holistic collection” approach, building on Robert Rausch’s early work. This methodology ensures that, in addition to multiple ultra frozen tissues (heart, liver, kidney, spleen) preserved for each traditional voucher, we also collect a full suite of symbionts (endoparasitic helminths, fleas, ticks, microbiomes, etc.) from each mammal. These fully integrated collections provide extraordinary opportunities to more fully examine species interactions and expand potential avenues of investigation into community ecology, conservation, host-pathogen interactions, and immunology – and more yet to be discovered. The holistic collecting of mammals is the most powerful means of developing the “intentional extended specimen” concept.