Hantavirus: Ecology and Disease in US, Chile, and Panama
Hantaviruses belong in the order Bunyavirales and the family Hantaviridae. These enveloped, negative-sense single stranded RNA viruses have unique tri-segmented (tripartite) genomes. Hantaviruses cause two human diseases: Hemorrhagic Fever with Renal Syndrome (HFRS), found in Europe, Asia, and Africa (Old World), or Hantavirus Cardiopulmonary Syndrome (HCPS), found in the Americas (New World).
Infection occurs through the inhalation of aerosolized rodent excrement (urine, feces, saliva). With the exception of a few cases in the Andes virus, human-to-human transmission does not occur. Sin Nombre Hantavirus was the first known HCPS in the Americas to infect humans.

The first hantavirus was isolated in 1978 in South Korea by Dr. Ho Wang Lee and Dr. Karl Johnson (1929-2023), former Adjunct Professor of Biology at the University of New Mexico. Dr. Johnson, founder of the CDC’s Special Pathogens branch, helped develop the modern BSL-4 lab and made significant contributions to tropical virology. At UNM, his research focused on hantavirus disease and ecology. He was a longstanding associate and vocal advocate for the MSB’s approach to the development of a spatially broad and temporally deep biorepository infrastructure for pathogen research.
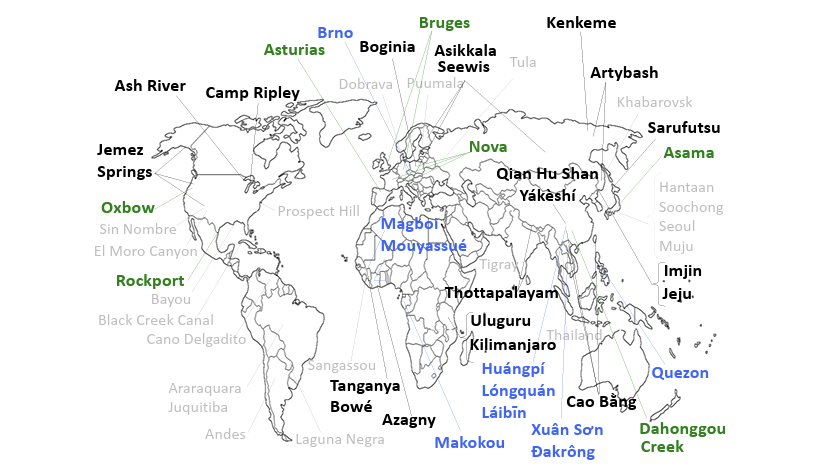
Hantaviruses were previously classified as rodent-borne viruses (roboviruses). Historically, research has primarily focused on rodent vectors, with less consideration given to the possibility that hantaviruses could be present in other mammal species.
Dr. Richard Yanagihara and colleagues probed MSB specimens to show in a series of papers that hantaviruses are found in non-rodent mammals: bats (Chiroptera), moles (Talpidae), and shrews (Soricidae). Bats as reservoir hosts for hantaviruses are a particularly important discovery, due to their close proximity to human populations.
In a 2020 paper, Dr. Yanagihara, Dr. Satoru Arai, and colleagues demonstrated the importance of archival tissue collections in understanding host diversification, host range, and viral genetic diversity in non-rodent hosts. They also noted that geographic distribution, which can be assessed through these collections, is vital in evaluating risk and predicting future viral outbreaks that could impact human populations.
Hantavirus Research — MSB Collaborations
Researchers affiliated with MSB have worked closely with Dr. Yanagihara, Satoru Arai, and others to discover novel hantaviruses in non-rodent vectors. These newly identified viruses cluster into host clades, span across five continents, and exhibit greater genetic diversity than those found in rodents. This points to a more complex evolutionary history of hantaviruses than previously understood. The cell cultures for these novel hantaviruses have yet to be isolated, so risk of human infection is presently unknown.

Dr. Blas Armien at the Instituto Conmemorativo Gorgas (Gorgas Memorial Institute; ICG), in Panama City, Panama. MSB’s collaboration with the ICG began in 2000, with numerous Panamanian field collection trips, the most recent in May 2024. The Division of Mammals maintains over 10,000 specimens, associated data, parasites, and tissues collected from these efforts, with many being examined for local hantaviruses.

The Chile Hantavirus Project, funded by the International Collaborations in Infectious Disease Research (ICIDR) program, spanned from 1999 through 2011, with over 7,000 specimens catalogued into the MSB’s online database, Arctos. Specimens are also archived in the Colección de Flora y Fauna Patricio Sánchez Reyes, maintained by the Pontificia Universidad Católica de Chile in Santiago, Chile. This collaboration has produced multiple publications utilizing specimens from this project, including a 2019 paper detailing the small mammals of Chile associated with human HCPS cases.
The Bradfute Lab, associated with UNM North Campus, engages in emerging viral pathogen research, particularly with hantaviruses, arenaviruses, and coronaviruses. In January 2025, the Bradfute Lab and the MSB published a paper detailing the taxonomic diversity of rodent-borne Sin Nombre Hantavirus.

Contemporary collaboration is spearheaded through the Museum and Emerging Pathogens in the Americas (MEPA), a network of >150 researchers from over 40 different organizations in the Americas. With disciplines ranging from public health, wildlife biology, veterinary, pathobiology, and more, this international network provides an important framework for the study of diseases, as well as the identification of future zoonoses that may emerge.



