Controlling Human Schistosomiasis
As noted in our mission statement, one of the goals of our division is to understand how the complexity of biotic environments influences the transmission of infectious diseases. Arguments have been made that disease transmission is favored in some cases, or hindered in others, in biotically rich environments. In cooperation of scientists from the Kenya Medical Research Institute in Nairobi, Kenya, Division of Parasitology scientists are investigating the impact of one form of biotic diversity – the number of digenetic trematode species present – on the transmission of the human parasite Schistosoma mansoni in a variety of different habitats in western Kenya.

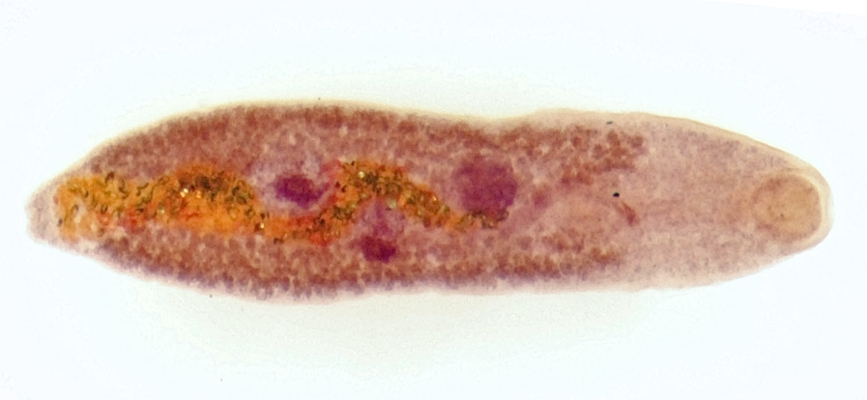
Adult worms of S. mansoni develop in the vascular system of humans, often children. The parasite’s eggs are passed in the feces and if they enter freshwater habitats, hatch and release ciliated miracidia, which seek, out and infect freshwater snails of a particular genus, Biomphalaria. One snail species, B. pfeifferi is particularly important for transmission of S. mansoni across much of Africa. Within the snail, the parasite undergoes an extensive period of asexual development, culminating in the production of fork-tailed cercariae, which are released into the water. The cercariae penetrate human skin to start the cycle anew. A single infected snail can produce hundreds of cercariae per day for weeks or, as some of our studies show, even as long as a year. Human schistosomiasis still afflicts over 200 million people, mostly in sub-Saharan Africa, and current control efforts are dangerously uni-dimensional, relying on extensive use of a single drug to treat infected people. Alternative forms of control are needed, particularly those targeting the parasite as it develops in its snail host, if more sustainable control is to be attained and the WHO’s goal of the elimination of human schistosomiasis as a public health problem by 2025 is to be achieved.
Ongoing studies in Kenya have revealed that along with S. mansoni, several additional species of digenetic trematodes also use the same snail, B. pfeifferi, for their larval development. These trematodes, just as with S. mansoni, have complex life cycles involving fish, amphibians, reptiles, birds or mammals as hosts for adult worms, but also rely on the snail for essential production of cercariae. In other words, biotically complex environments that favor both the persistence of these other host and trematode species, potentially impact the transmission of the human parasite, S. mansoni. Indeed, it is known that the larval stages of different trematode species compete with and/or prey upon one another if found together in the same snail.

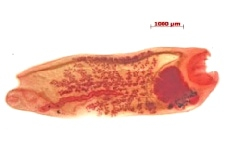
Our first goal has been to first identify many of the trematode species that co-inhabit B. pfeifferi along with S. mansoni, and Division of Parasitology scientists have been actively engaged in this endeavor. For example, we have found at least 10 different species of trematodes to use B. pfeifferi snails in streams that support S. mansoni transmission in western Kenya. Additional studies of amphistome trematodes, which infect local domestic ruminants and are heavy users of these same streams (Figure 2), have revealed over 15 species of amphistomes alone to be present. Several of these amphistome species use Bulinus snails as obligatory hosts. This is noteworthy because species of Bulinus are the required snail hosts for another important human parasite in Africa, Schistosoma haematobium the causative agent of urinary schistosomiasis.

We have initiated a series of experiments to examine the extent to which the larvae of other trematodes can interfere with the larval development of S. mansoni. For instance, amphistome species produce larval forms within snails called rediae, which can potentially attack and consume schistosome sporocysts. Indeed, in habitats where schistosomes and amphistomes are both common, a dearth of double infections is noted, suggestive that schistosomes and amphistomes have antagonistic interactions with one another in snails. Experimental studies have shown that snails with established amphistome infections are not readily infected with S. mansoni. Additional studies are underway using echinostome trematodes, which are normally transmitted by wild bird populations. Birds often accumulate in significant numbers around the shoreline at Lake Victoria where people also congregate (Figure 3), and the ability of the echinostomes to infect nearby Biomphalaria snails with echinostomes could well have the effect of protecting people from S. mansoni infections. Our goal is to exploit the biodiversity present in such habitats – in the form of diverse trematode species and the competitive interactions they have with schistosomes – to diminish the number of S. mansoni infections in snails, thereby limiting the number of human-infecting cercariae they produce. This entire approach depends on characterization of the diversity of the parasites present, and establishing a collection within MSB that can be accessed by others so they can understand the nature of this diversity and how it might be exploited elsewhere in the world for trematode control.